Cryptorchidism is as common as type 2 diabetes or celiac disease. Boys with congenital cryptorchidism are atincreased risk of infertility and testicular cancer. Zika syndrome, which affects pregnant women, is associated with ahigh incidence of undescended testes in the infant, accompanied by epididymal anomalies. Zika and influenza virusinfections during pregnancy trigger a strong anti-inflammatory immune response and elevated estradiol levels.Elevated estradiol and α-fetoprotein in syncytiotrophoblasts from women who have given birth to cryptorchid boysare indicative of increased estradiol levels in the fetus. Here, I present a hypothesis that hypogonadotropichypogonadism, cryptorchidism, and retarded epididymal development may be due to elevated fetal estradiol levelscaused by viral infection during pregnancy. Download complete article
Category: Cryptorchidism
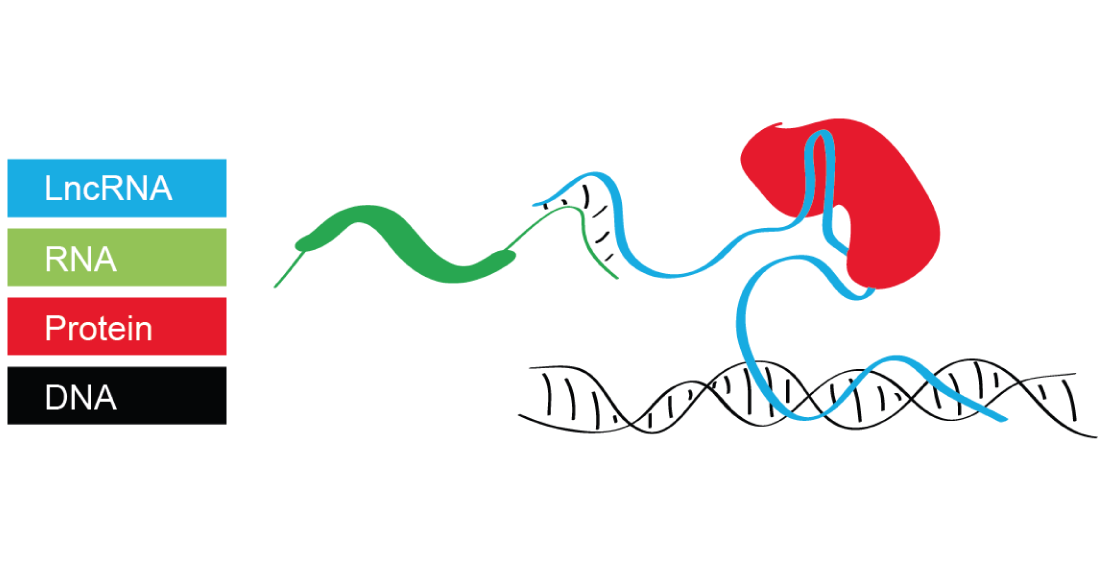
Testicular expression of long non–coding RNAs is affected by curative GnRHa treatment of cryptorchidism
Background: Cryptorchidism is a frequent endocrinopathy in boys that has been associated with an increased riskof developing testicular cancer and infertility. The condition is curable by combined surgery and hormonaltreatment during early pre-pubertal stages using gonadotropin releasing hormone agonist (GnRHa). However,whether the treatment also alters the expression of testicular long non-coding RNAs (lncRNAs) is unknown. To gaininsight into the effect of GnRHa on testicular lncRNA levels, we re-analyzed an expression dataset generated fromtesticular biopsies obtained during orchidopexy for bilateral cryptorchidism.Results: We identified EGFR-AS1, Linc-ROR, LINC00221, LINC00261, LINC00282, LINC00293, LINC00303, LINC00898,LINC00994, LINC01121, LINC01553, and MTOR-AS1 as potentially relevant for the stimulation of cell proliferationmediated by GnRHa based on their direct or indirect association with rapidly dividing cells in normal andpathological tissues. Surgery alone failed to alter the expression of these transcripts.Conclusion: Given that lncRNAs can cooperate with chromatin-modifying enzymes to promote epigeneticregulation of genes, GnRHa treatment may act as a surrogate for mini-puberty by triggering the differentiation ofAd spermatogonia via lncRNA-mediated epigenetic effects. Our work provides additional molecular evidence that infertility and azoosper
Download complete article
DMRTC2, PAX7, BRACHYURY/T and TERT Are Implicated in Male Germ Cell Development Following Curative Hormone Treatment for Cryptorchidism-Induced Infertility
Defective mini-puberty results in insufficient testosterone secretion that impairs the differentiation of gonocytes into dark-type (Ad) spermatogonia. The differentiation of gonocytes into Ad spermatogonia can be induced by administration of the gonadotropin-releasing hormone agonist, GnRHa (Buserelin, INN)). Nothing is known about the mechanism that underlies successful GnRHa treatment in the germ cells.
Download complete article